Published on August 8, 2025 9:21 PM GMT
In the past 3 entries in my consciousness sequence I was focused on conscious sensory perception — the difference between sense data that we subjectively “notice” or “are aware of”, vs. sense data that we are exposed to, and which may affect us, but without our knowledge.
This is one of Erik Hoel’s three common-sense characteristics of consciousness. We are conscious of a thing when we have a subjective experience of noticing or perceiving it; we are unconscious of the same thing when we have no such experience.
Another common-sense characteristic of consciousness is awakeness (or “arousal”). Ordinary waking life is a conscious state; dreamless sleep, anaesthesia, and coma are unconscious states.
In unconscious states, the individual is largely unresponsive to outside stimuli, cannot communicate, and will wake with no memories of having experienced anything while unconscious.
As far as we can tell, in an unconscious state, the individual has no conscious/subjective perceptions of anything. If being conscious “of something” means that it enters a sort of “workspace” or “field of awareness” where we can notice/perceive it, then an unconscious state means an empty workspace or field of awareness.
So, what’s different in the brain during an unconscious or less-conscious state?
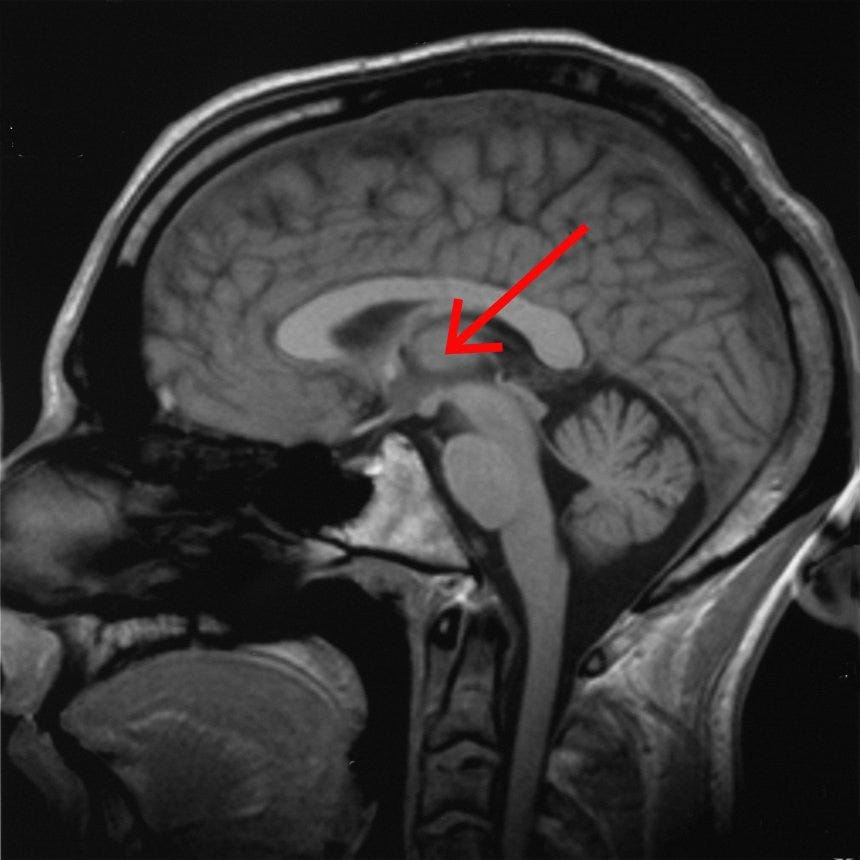
At a first approximation, it’s the thalamus, stupid.
During less-conscious states, the thalamus is less active, more inclined to slow synchronized firing than fast desynchronized firing, and less “functionally connected to” (i.e. activity correlates less well with) other brain regions, particularly in the cortex.
Interestingly, my last (imperfect) candidate for a brain region critical for conscious sensory perception was a particular nucleus in the thalamus.
Neural Correlates of Disorders of Consciousness: It’s The Thalamus (and Friends)
“Disorders of Consciousness” (DOC) is a catch-all term that includes things like coma, minimally conscious states, vegetative states, and so on.
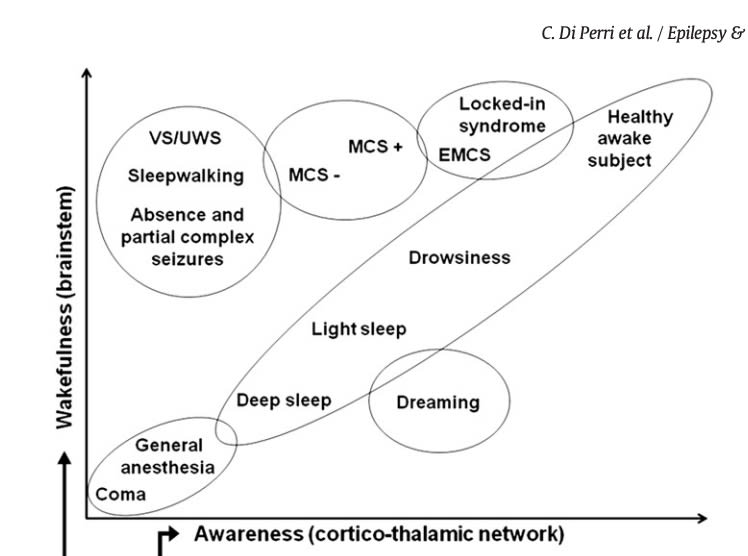
Interestingly, there are two axes on which disorders of consciousness differ from the normal healthy waking state: arousal and awareness.1
In a vegetative state the patient is unresponsive to their surroundings, but still has open eyes and sleep-wake cycles. They are, in some sense, awake…but appear to not be aware of anything around them. (Typically, a coma that doesn’t end earlier will turn into a vegetative state after a few weeks.)
Similarly, in an absence seizure, some degree of wakefulness is still present (open eyes, standing, etc) but no response to their surroundings. In both absence seizures and vegetative states, patients typically have no memory of the experience after they recover, consistent with them having no conscious subjective experiences during the “low awareness state.”
While lesions in many places can cause disorders of consciousness, the thalamus is a common lesion site, as well as a network of fronto-parietal cortex sites known as the default mode network (which is typically active during self-related thought processes like mind-wandering and autobiographical memory.)
In a meta-analysis of fMRI studies of disorders of consciousness, covering a total of 687 patients and 637 healthy controls, activity in the DOC patients was significantly reduced in regions within the default mode network, including2:
bilateral medial dorsal nucleus of the thalamus
left cingulate
posterior cingulate
precuneus
middle frontal and medial temporal gyri
A subsequent study similarly found that clinical measures of awareness, in disorders of consciousness, correlated with the degree of damage to the thalamus.3
The vegetative state, in particular, is overwhelmingly likely to coincide with damage to the thalamus (80% of patients).4
Interestingly, you can get brain activity in response to painful stimuli and sounds in vegetative state patients — but only in brainstem, thalamus, and the “primary” somatosensory and auditory cortices, not in the “secondary” sensory-processing areas that typically correlate with conscious sensory perception and more complex pattern-recognition.5
From an EEG point of view, patients in comas or vegetative states usually have only very slow-wave activity, delta or theta waves, similar to deep sleep. Brain-dead patients have no EEG activity; “locked-in” patients, who are conscious and can communicate by thought alone, but can’t move, have normal EEGs.6
Neural Correlates of Anaesthesia: Yep, Still The Thalamus
During propofol anaesthesia, EEG waves slow down and increase in amplitude, first to alpha waves, and then to delta and theta waves.7
Under PET imaging, lots of regions in the brain show lower metabolism and blood flow during anaesthesia, but particularly the thalamus and cortex.8
You get the same results with fMRI imaging: reliably, less blood flow to the thalamus, with a strong dose-response relationship — the more anaesthetic, the less thalamic blood flow. 9
Neural Correlates of Deep Sleep: Once Again, That’s The Thalamus
The classic neural correlate of sleep is the EEG.
Beta waves for alert (or dreaming) states, alpha waves for calm or drowsy states, and delta and theta waves for deep sleep.
These are brain-wide patterns of electrical activity, averaged across the whole brain volume and measured with scalp electrodes.
But there’s some evidence that the thalamus leads the whole-brain transition from fast uncorrelated spiking (and thus high-frequency EEG patterns) to slower and more synchronized brain waves (and thus the lower-frequency delta and theta waves.)
During the normal waking state, neurons in the thalamus spike “tonically” (at a sustained, high baseline rate). By contrast, during sleep, the thalamus “sets the pace” for the slow “sleep spindle” oscillations characteristic of entry into deep non-REM sleep. It’s actually T-type calcium channels in the thalamus, specifically, that entrain these sleep spindles, and you can induce them artificially in rodents with optogenetics.10 Both sleep spindles and slow-wave oscillations in sleeping humans happen first in thalamus and only afterwards in the cortex.11
Other regions, like the brainstem and hypothalamus, are also highly involved in the transition between sleep and waking. You can induce sleep in a cat with pulsed electrode stimulation to lots of parts of the brain, including the basal forebrain12 , the cortex13, and the thalamus14.
But, for instance, there are particular neurons in the thalamus active during the process of loud sounds awaking animals from deep sleep, and optogenetic stimulation of those neurons will wake the animal up.15
It seems that “lower” regions like the hypothalamus and brainstem connect to the thalamus, and have some of their effects on sleep through the thalamus. So while it’s not the only region important for sleep, it might be the proximate controller of sleep and slow-wave firing patterns in the rest of the brain.
Upshots
This is a very high-level overview; we haven’t touched on where or how the thalamus affects states of consciousness, or any DOC-adjacent conditions like confusional states.
But we do have a very clear and consistent signal that virtually all unconscious states involve alterations in the thalamus, which is intriguing because the thalamus is also the connecting hub that integrates between different sensory perceptions and the cortical regions that process them.
Unfortunately, we don’t (yet) seem to have reliable results at bringing people out of comatose states by stimulating the thalamus1617.
But we might learn more about how consciousness works by zooming in on this region. As a rough heuristic, the (human) cortex tends to be very flexible, while subcortical brain structures are less so; if any function is going to be anatomically “hard-coded”, it’ll probably be subcortical. It’s also more likely that more “ancient” subcortical structures will be similar between humans and animals. So if the toolbox of neuroscience is to be helpful for making sense of consciousness, we should be more hopeful about subcortical sites. And luckily we seem to have one!
Di Perri, Carol, et al. "Functional neuroanatomy of disorders of consciousness." Epilepsy & Behavior 30 (2014): 28-32.
Hannawi, Yousef, et al. "Resting brain activity in disorders of consciousness: a systematic review and meta-analysis." Neurology 84.12 (2015): 1272-1280.
Lutkenhoff, Evan S., et al. "Thalamic and extrathalamic mechanisms of consciousness after severe brain injury." Annals of neurology 78.1 (2015): 68-76.
Adams, J. Hume, D. I. Graham, and Bryan Jennett. "The neuropathology of the vegetative state after an acute brain insult." Brain 123.7 (2000): 1327-1338.
Laureys, Steven. "The neural correlate of (un) awareness: lessons from the vegetative state." Trends in cognitive sciences 9.12 (2005): 556-559.
Bernat, James L. "Chronic disorders of consciousness." The Lancet 367.9517 (2006): 1181-1192.
Hagihira, S. "Changes in the electroencephalogram during anaesthesia and their physiological basis." British journal of anaesthesia 115.suppl_1 (2015): i27-i31.
Menon, D. M. "Editorial I: Mapping the anatomy of unconsciousness—imaging anaesthetic action in the brain." British Journal of Anaesthesia 86.5 (2001): 607-617.
Song, Xiao-xing, and Bu-wei Yu. "Anesthetic effects of propofol in the healthy human brain: functional imaging evidence." Journal of anesthesia 29.2 (2015): 279-288.
Whyte, Christopher J., et al. "Thalamic contributions to the state and contents of consciousness." Neuron 112.10 (2024): 1611-1625.
Schreiner, Thomas, et al. "The human thalamus orchestrates neocortical oscillations during NREM sleep." Nature communications 13.1 (2022): 5231.
Sterman, M. Bo, and CD13916976 Clemente. "Forebrain inhibitory mechanisms: sleep patterns induced by basal forebrain stimulation in the behaving cat." Experimental neurology 6.2 (1962): 103-117.
Penaloza-Rojas, J. H., M. Elterman, and N. Olmos. "Sleep induced by cortical stimulation." Experimental neurology 10.2 (1964): 140-147.
Akert, Konrad, W. P. Koella, and R. Hess Jr. "Sleep produced by electrical stimulation of the thalamus." American Journal of Physiology-Legacy Content 168.1 (1951): 260-267.
Shin, Anna, et al. "A brainstem-to-mediodorsal thalamic pathway mediates sound-induced arousal from slow-wave sleep." Current Biology 33.5 (2023): 875-885.
Bergeron, David, et al. "Central thalamic deep brain stimulation for disorders of consciousness: an individual participant data meta-analysis." Journal of Neurosurgery 1.aop (2025): 1-10.
Cain, Joshua A., et al. "Ultrasonic thalamic stimulation in chronic disorders of consciousness." Brain Stimulation: Basic, Translational, and Clinical Research in Neuromodulation 14.2 (2021): 301-303.
Discuss