A new method for generating high-energy proton beams could one day improve the precision of proton therapy for treating cancer. Developed by an international research collaboration headed up at the National University of Singapore, the technique involves accelerating H2+ ions and then using a novel two-dimensional carbon membrane to split the high-energy ion beam into beams of protons.
One obstacle when accelerating large numbers of protons together is that they all carry the same positive charge and thus naturally repel each other. This so-called space–charge effect makes it difficult to keep the beam tight and focused.
“By accelerating H₂⁺ ions instead of single protons, the particles don’t repel each other as strongly,” says project leader Jiong Lu. “This enables delivery of proton beam currents up to an order of magnitude higher than those from existing cyclotrons.”
Lu explains that a high-current proton beam can deliver more protons in a shorter time, making proton treatments quicker, more precise and targeting tumours more effectively. Such a proton beam could also be employed in FLASH therapy, an emerging treatment that delivers therapeutic radiation at ultrahigh dose rates to reduce normal tissue toxicity while preserving anti-tumour activity.
Industry-compatible fabrication
The key to this technique lies in the choice of an optimal membrane with which to split the H₂⁺ ions. For this task, Lu and colleagues developed a new material – ultraclean monolayer amorphous carbon (UC-MAC). MAC is similar in structure to graphene, but instead of an ordered honeycomb structure of hexagonal rings, it contains a disordered mix of five-, six-, seven and eight-membered carbon rings. This disorder creates angstrom-scale pores in the films, which can be used to split the H₂⁺ ions into protons as they pass through.
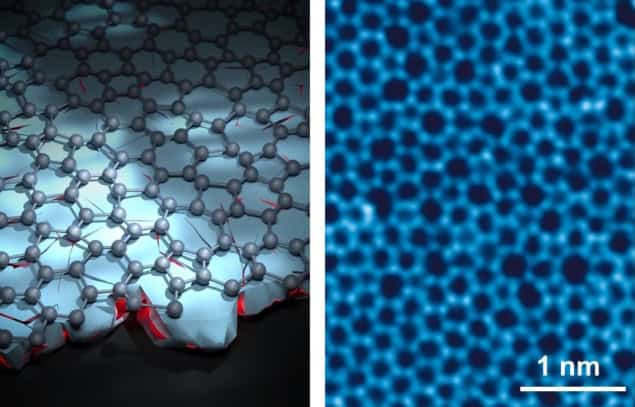
Scaling the manufacture of ultrathin MAC films, however, has previously proved challenging, with no industrial synthesis method available. To address this problem, the researchers proposed a new fabrication approach in which the emergence of long-range order in the material is suppressed, not by the conventional approach of low-temperature growth, but by a novel disorder-to-disorder (DTD) strategy.
DTD synthesis uses plasma-enhanced chemical vapor deposition (CVD) to create a MAC film on a copper substrate containing numerous nanoscale crystalline grains. This disordered substrate induces high levels of randomized nucleation in the carbon layer and disrupts long-range order. The approach enabled wafer-scale (8-inch) production of UC-MAC films within just 3 s – an order of magnitude faster than conventional CVD methods.
Disorder creates precision
To assess the ability of UC-MAC to split H₂⁺ ions into protons, the researchers generated a high-energy H2+ nanobeam and focused it onto a freestanding two-dimensional UC-MAC crystal. This resulted in the ion beam splitting to create high-precision proton beams. For comparison they repeated the experiment (with beam current stabilities controlled within 10%) using single-crystal graphene, non-clean MAC with metal impurities and commercial carbon thin films (8 nm).
Measuring double-proton events – in which two proton signals are detected from a single H2+ ion splitting – as an indicator for proton scattering revealed that the UC-MAC membrane produced far fewer unwanted scattered protons than the other films. Ion splitting using UC-MAC resulted in about 47 double-proton events over a 20 s collection time, while the graphene film exhibited roughly twice this number and the non-clean MAC slightly more. The carbon thin film generated around 46 times more scattering events.
The researchers point out that the reduced double-proton events in UC-MAC “demonstrate its superior ability to minimize proton scattering compared with commercial materials”. They note that as well as UC-MAC creating a superior quality proton beam, the technique provides control over the splitting rate, with yields ranging from 88.8 to 296.0 proton events per second per detector.
“Using UC-MAC to split H₂⁺ produces a highly sharpened, high-energy proton beam with minimal scattering and high spatial precision,” says Lu. “This allows more precise targeting in proton therapy – particularly for tumours in delicate or critical organs.”
“Building on our achievement of producing proton beams with greatly reduced scattering, our team is now developing single molecule ion reaction platforms based on two-dimensional amorphous materials using high-energy ion nanobeam systems,” he tells Physics World. “Our goal is to make proton beams for cancer therapy even more precise, more affordable and easier to use in clinical settings.”
The study is reported in Nature Nanotechnology.
The post Amorphous carbon membrane creates precision proton beams for cancer therapy appeared first on Physics World.

