Published on April 19, 2025 6:37 PM GMT
This post summarizes some of the research I have been doing forBootstrap Bio AKA kman andGenesmith
TL;DR
- We currently don’t have a list of all imprinted genes that areimportant in human development, but further long-read sequencing inadults and in the human placenta is going to get us close to thatgoal.Measuring DNA methylation in a low input DNA context is reallyannoying, because usually we can’t amplify methylated DNA. I explorethe only way that appears tractable to me to amplify methylated DNA by combining Phi29 and DNMT1. This could be useful for building an atlas of early embryodevelopment and for epigenetic preimplantation embryo screening.Correct imprinting seems particularly achievable in Hulksperm
Epigenetics and imprinting
This post assumes basic familiarity with epigenetics. The next paragraphis a 1-paragraph summary, but I recommend readingthesetwoexcellent posts on epigenetics. If you know what DNA methylation, DNMT1and imprinting are, you can safely skip those posts. You might also justdo fine by using the AI-generated glossary.
The short explanation is that DNA methylation consists of small chemicalmarks on DNA that change which proteins can bind well to it. Togetherwith other chemical marks onhistones, which are “spools”that DNA is rolled around, these marks are how a cell knows that it is askin cell, rather than a neuron. Imprinting is a phenomenon where thetwo sets of chromosomes in early mammal embryos behave differentlydepending on if they were inherited from the father or the mother side.Imprinting appears to be mostly due to differences in DNA methylationand histone marks. DNA methylation and histone marks are maintainedacross cell replications through an array of specific enzymes that bothadd, maintain (DNMT enzymes in the case of methylation) and remove marks(Tet enzymes in the case of DNA methylation). Approximate maintenance ofmethylation is achieved by a balance between the actions of theseenzymes in ways that aren’t fully understood yet.
Why we need to know all imprinting regions and genes
All strong methods of germline engineering require turning culturedcells that are not fully totipotent, back into totipotent or naivepluripotent stem cells[1] that an embryo can grow from. We might get tosuch a state through differentmechanisms,including in vitro oogenesis, spermatogenesis or maybe we are able tofind a cocktail that turns cells naive directly. While the Yamanakafactors make it possible to turn cells into a more stem-cell like state,they are not fully totipotent. One distinction that is often made hereis between naive and primed iPSCs. Naive iPSCs share somecharacteristics, but the most important one is that they can specializeinto cells from all three germ layers and are similar to cells in theblastocyst. Currently all methods to create naive human pluripotent stemcells are prone to loss of imprinting. If we properly imitate oogenesisand spermatogenesis in vitro, imprints are also erased. So in both casesit is important that we know of and understand all imprints in order topreserve or reestablish them.
Loss of imprinting tends to be really bad for embryos and havenon-negligible effects. For example loss of paternal ZDBF2 imprinting is associated with a20% lower weight in 2 week year old mice and this effect persists inweaker form throughout development![2] Confusingly though, ZDBF2 isfound on chromosome 2 and presumably if ZDBF2 has such large effects itwould have been seen as a pattern in UPD 2cases? But perhaps UPD2 is toorare for the same doctor to have seen multiple patients to connect thedots? I think it’s also plausible that imprinting generally has largereffects in mice. In mice there seems to be stronger competition betweenpaternal and maternal line due to being less monogamous than humans (Onesign of this is that IGF2R is always imprinted in mice, but onlypolymorphically imprinted in humans).
Thus if we want to make healthy embryos, we need to make sure that ALLimportant imprints are correct in early embryos.[3] For this goal, itwould be great if we had a list of all existing imprinted genes andtheir imprinting control regions, so we can make sure currentlydeveloped techniques to maintain imprinting don’t overlook any crucialones. In the rest of this post, I will do a deep dive through existingpapers, to explain how far we are in documenting all existing imprintedgenes and what methods we might use to better screen for them.
How imprinted genes work and how we measure them
So there are about ~200 imprinted genes. At least in mice the patternseems to be that there is a bimodal distribution between genes that areheavily biased in expression (close to 0 expression from 1 parent) andthe regular genes which are expressed in roughly equal ratios. There aresome genes that are only a little biased (think 52/48) and these tend tooccur close to existing imprinting regions (Edwards et al., 2023). Ithink it is fair for the purposes of germline engineering to treatlittle biased genes as not imprinted.
In humans, it appears that all imprinted genes are ultimately regulatedthrough some differentially methylated region (DMR). A DMR in thiscontext is a region that is methylated in the chromosome inherited fromone parent, but not the other. When there are differences in methylationbetween different cell types, those are also often referred to as DMRs,but those are not interesting to us in this post.
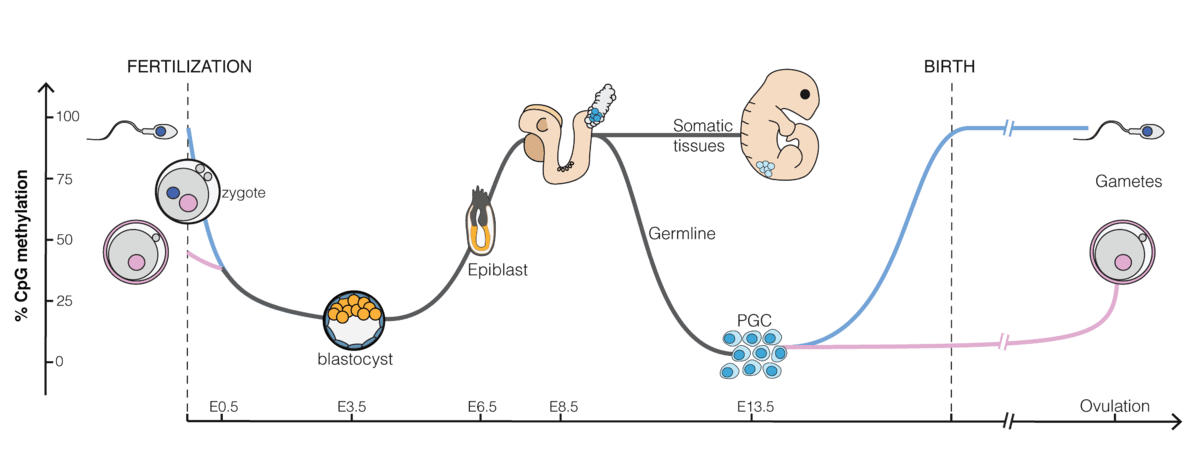
As you can see in the image below from wikipedia, methylation tends tobe low in early embryonic development and then increasepost-implantation. It is not shown in this image, but methylation tendsto increase more in the inner cell mass compared to trophoblasts whichstay more unmethylated. For this reason there are some differentiallymethylated regions that are only transient in the embryo (tDMRs), butthat stick around in the placenta.
The majority of methylated regions only become differentially methylatedafter fertilization (somatic DMRs or sDMRs) caused by some nearby DMRthat was inherited from the oocyte or sperm (germline DMRs or gDMRs). Inmice, some sDMRs are established through the histone mark H3K27me3 setin the oocyte which then become methylated in preimplantation embryos.That methylation is then lost again in postimplantation embryos, butpersists in the placenta. Since these types of DMRs are not followingthe “canonical” pattern of an imprint established through a methylatedgDMR, they are called non-canonical imprints. It is possible that thereare imprints in humans that are established through histones, as in thenon-canonical imprints in mice. But if that’s the case it is probably arare phenomenon, otherwise Daskeviciute et al. (2025) would have foundone of these DMRs when they went explicitly looking for them.
We have identified some of the proteins that are involved in maintainingimprints. For example ZDBF2 and ZNF445 appear necessary to maintainmaternally methylated DMRs. CTCF and the histone mark H3K4me3 can helpmaintain DMRs unmethylated.
Have we identified all imprinted genes?
One natural question to ask if we are interested in germline engineeringis if we have already identified all imprinted genes or if we at leastknow all imprinted genes that are crucial for development.
One of the first ways how imprinted genes were discovered is throughpatients with uniparentaldisomy, a raregenetic disorder where for a specific chromosome the patient hasinherited two copies of a chromosome from the same parent, but none fromthe other (often as the result of a “rescued” trisomy). For more info,see my post onUPD.
Unfortunately, there isn’t one well maintained list for all knownimprints. But combining the list fromgeneimprint.com with the list from Tucciet al. (2019) is pretty close to such a list. For learning about how weknow specific genes are imprinted I would also recommend the Catalogueof Parent of Origin Effectsover geneimprint.com, because it tends tocite more sources and includes more rational for why a gene was includedor not. The genes found in these lists have been identified eitherthrough UPD disorders or methylation and RNA sequencing in both mice andhumans.
Here’s how to find imprinted genes through RNA sequencing: Find RNAtranscripts that are more often expressed from one chromosome ratherthan another. It is possible to identify if RNA fragments are fromdifferent chromosomes through SNPs that are different between maternaland paternal chromosome. If we have DNA data from the parents we caneven identify if they are maternally or paternally imprinted as was doneby Jadhav et al. (2019).
Some imprinted genes might be hard to track through RNA expression inhumans, specifically for genes that are only imprinted in some specifickind of neuron or similar.
If the gene is also not imprinted in mice, one way how we could stillidentify such an imprint is through screening the entire genome fordifferentially methylated regions with high coverage epigeneticsequencing. If we do such screening for a lot of diverse cell types, I’dbe relatively confident that we did not miss any imprinted genes.
Well known DMRs tend to be around one thousand pairs long and containabout 50 CpG sites and are about ~0-10% methylated on one chromosome and~90% on the other (Monk et al., 2018). These are easy to identifythrough bisulfite sequencing by searching for long stretches that areabout 50% methylated on average. Obviously there could be the problemthat the important DMRs that we haven’t discovered are shorter orperhaps they are on average 70% methylated, because one chromosome isn’tfully unmethylated. There is really no way we can be sure how many suchDMRs there are without drowning in false positives as long as we do notphase bisulfite reads by chromosome (like Zink et al. (2018) does).
Fortunately, long-read sequencing has recently become more economicaland long-read sequencing allows us to both read methylation while alsoallowing us to phase DNA reads. We also have long-read DNA data that hashigher coverage than any bisulfite sequencing that has ever been done todate. Using phased nanopore reads Akbari et al. (n.d.) was able toidentify about 50 novel large differentially methylated regions. All ofthese novel regions seemed to be polymorphic and not differentiallymethylated in all individuals. Akbari et al. (n.d.) also identify 17 ofthese imprints to be conserved in mice and monkeys. So I don’t think itis off the table that these imprinted regions are essential early indevelopment, in the brain or the placenta, but appear “polymorphic” inblood and other tissues. What is also suspicious is that Akbari et al.(n.d.) identify some imprints (like ZNF714) as somatic DMRs because theyshow less than 50% methylation in blastocysts. My best guess is thatZNF714 actually has a gDMR on its promoter that just appears to be alittle less methylated in the blastocyst stage, but still managed tostay maintained.
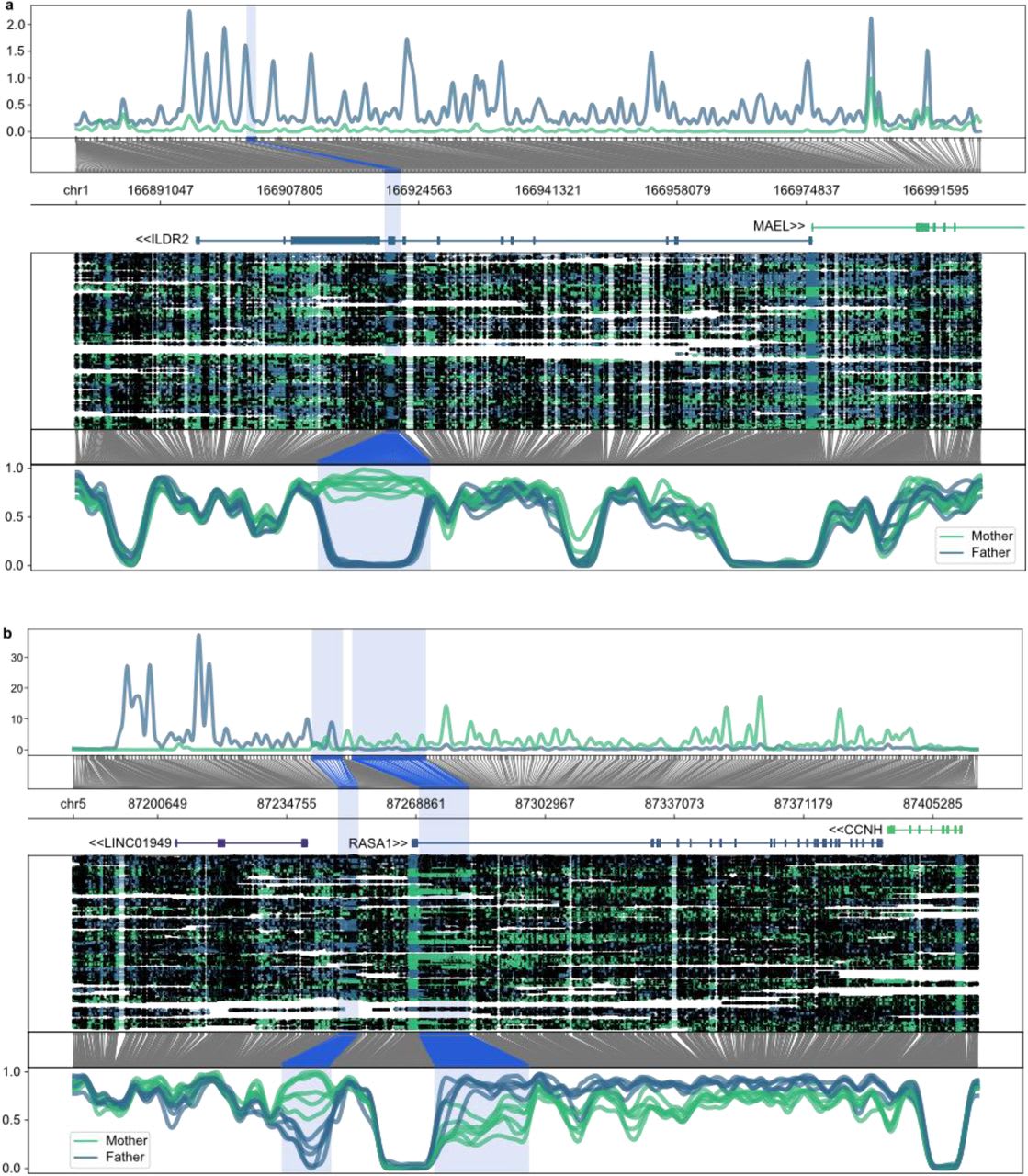
Kindlova et al. (2023) uses phased parent of origin assigned nanoporereads to investigate imprinting in 8 human placentas. Kindlova et al.(2023) find two DMRs on genes not previously known to be imprinted, thatthey describe in more detail in their paper, but supplementary table 4lists at least another ~90 such DMRs that weren’t identified in previousstudies that show affinity for ZFP57. It’s possible that a lot of theseare false positives, but some of them are probably real. Additionalplacenta samples will hopefully show which of these genuine in thefuture.
An image of the two novel DMRs discovered by Kindlova et al. (2023). The upper image shows the DMR on an exon of ILDR2 and the lower image shows the atypically looking DMR around the RASA1 promoter.
Fixing paternal imprinting might be easier than fixing maternal imprinting
Most imprints are maternally methylated and paternally unmethylated.This means creating Hulkspermwith correct imprinting might be comparatively easy, because we justerase all imprints by demethylating the cell on the way of turning itinto a spermatogonial stem cell like cell and before transfering theminto someone’s testicles, we only need to make sure that all imprintsare properly demethylated and then perhaps using some more targetedstrategy, like epigeneticCrispr to deal withthe few remaining paternally methylated imprints.[4]
PCR for methylated DNA
Both for better understanding early embryos and for preimplantationscreening of embryos, better methods of measuring methylation would beuseful. Working with embryos means we are working with little DNA. 1cell has about 6 picograms of DNA. Illumina sequencing methods need~1000x more DNA than that to get adequate coverage and long-readsequencing methods have even higher inputrequirements.
The reason for this difficulty is that methylated DNA loses itsmethylation when amplified through common PCR methods. So traditionallythe only way to read methylation is to chemically convert unmethylatedcytosine into uracil (which both DNA polymerases and DNA sequencingmethods read as thymidine) before amplifying DNA for downstreamsequencing. The way this conversion has been done for the last 30 yearsis by treating DNA with sodium bisulfite. Not only does this degrade DNA(Tanaka & Okamoto, 2007), but it also reduces the complexity of the DNAsequence, making it harder to align reads. I have no lab experience andrudimentary chemistry knowledge, but a number often cited for how muchDNA is lost through bisulfite treatment range from 70%-95% and there aresome papers claiming to reduce the loss down to 25-35% under idealconditions (Rajput et al., 2012; Yi et al., 2017). Recently, methods toconvert the DNA via enzymes have also sprung up. Chatterton et al.(2023) try this in a single cell sequencing setting, but they end uplosing more DNA through enzymatic conversion than through regularbisulfite sequencing. Overall both methods do not solve the problem thatany procedure that we perform before amplifying the DNA is going to leadto unrecoverable losses in breadth of coverage.
So for this reason, if we want breadth of coverage with ultra-low inputDNA, we need a method to amplify our methylated DNA. I see one tractableway to do this that hasn’t been extensively tried before: Since ourhuman cells do it using the enzyme DNMT1 and the reagent SAM. For bothof these there don’t exist alternative natural proteins that canwithstand the high temperature during regular PCR, since methylatedcytosine aminates too often at high temperatures, so bacteria that canwithstand high temperatures use different chemical modifications to DNA.So we can only use polymerase that work well under low temperature(<40°C), which narrows down the DNA polymerase we can use toPhi29.[5] Liu et al. (2020) tried and patented[6] this idea. From theirFigure3a and S10 I infer that DNMT1 seems to do de-novo methylation at arate of 1-2% per replication and DNMT1 seems to fail to maintainmethylation a similar fraction of the time.[7] That is a high falsepositive and false negative rate, but not fatally so if we get morecoverage in return.[8] Overall their results are confusing in someareas, so I remain unsure if this method is any good. For example, Liuet al. (2020) get lower breadth coverage out of their amplified 10pgsamples, compared to their unamplified controls.[9]
If it turns out the idea above isn’t really tractable or Liu et al.(2020) aren’t willing to license their idea at a reasonable price, mybest guess is that methylation sequencing isn’t going to be highcoverage enough to be of good use for pre-implantation embryo screeningand (long or short-read) RNA sequencing might be more useful¯\(ツ)/¯. Possibly the enzyme conversion methodTAPScould still be worth it once commercially available, since it onlyconverts methylated Cs to Uracil and thus keeps most of the sequencecomplexity intact. Thisblogpostalso goes through some more intractable methylation sequencingstrategies.
Thoughts on the role of RNA in imprinting
- Long non-coding RNA
- The textbook example for the role of RNA in epigenetics is theXist gene, which silences one of the X chromosomes in humansand other mammals.Such long non-coding RNAs are sometimes used in imprintingregions to suppress multiple adjacent genes in cis. Prototypicalexamples are KCNQ1OT1 and Airn in mice (and presumablyKCNQ1OT1 functions similarly in humans). In fact, Xist appearsto be imprinted in mice, leading to mostly the paternalX-chromosome being inactivated in the placenta[10].I don’t know of any cases where the initial methylationvanishes, but the expression is still long-term imprinted.Seeing an example like that would be really interesting, but sofar I haven’t seen one and it seems long-term imprinting isalways maintained through methylation.People have been measuring these non-coding RNAs and there arenot THAT many of them around. If one of them was acting likememory in some imprint that doesn’t have methylation involvedsimilar to Xist, we would know that long non-coding RNA.
- There are examples of direct trans effects of an imprint onthe other allele, where the paternal allele produces anantisense microRNA that is breaking down the maternal mRNA forthat imprinted gene (Haig & Mainieri, 2020). There arepresumably more examples like this. We should not expectimprints to only have cis effects.
Some useful datasets and resources if someone else wants to look further into imprinting and epigenetics in the future
I learned the hard way that epigenetics is too new a field to get a goodunderstanding by reading thetextbook,because being 13 years out of date is in fact making a big difference.For example for learning about histones and chromatin states, I wouldnow recommend this YouTubevideoand after that just the wikipediaarticle.
When checking if a gene is a somatic or a germline differentiallymethylated region, it is important to have data about CpG methylationfor both sperm and oocytes. Unfortunately oocytes are really expensiveand even if studies end up using a handful of oocytes for bisulfite seq,the coverage per Oocyte is abysmal (1-10% of the genome are covered, for42 oocytes, 12 oocytes or 9 oocytes (Hernandez Mora et al., 2023; Li etal., 2018; Zhu et al., 2018)). One exception is Okae et al. (2014),which used about 200 oocytes, but they didn’t provide me their processeddata when I emailed them and their raw reads are in the JapanGenotype-Phenotype Archive, which is impossible to access if you don’thave an ethics board that has approved of whatever you want to do withthat data. Fortunately, Akbari et al. (n.d.) used Okae et al.’s (2014)data to identify gDMRs in their paper and provided all the aggregatedmethylation data of those oocytes in the data repository associatedwith that study! That same datarepository also contains a lotof other useful files for understanding imprinting, including bothhistone and methylation marks phased and separated by parent of origin.You can open all of these files in either igv,ucsc or the genome browser of your choice.
I also found some more phased methylation reads on the 1000 genomesproject’s AWS bucket, that mightbe worth downloading.[11]
I can also recommend to read through some public peer review files,which gives a better impression what the state of the art is foracademic outsiders like myself. The peerreviewof Akbari et al. (n.d.)and ofsomeother studies is publiclyavailable.
References
Akbari, V., Garant, J.-M., O’Neill, K., Pandoh, P., Moore, R., Marra, M.A., Hirst, M., & Jones, S. J. (n.d.). Genome-wide detection of imprinteddifferentially methylated regions using nanopore sequencing. eLife,11, e77898. https://doi.org/10.7554/eLife.77898
Chatterton, Z., Lamichhane, P., Ahmadi Rastegar, D., Fitzpatrick, L.,Lebhar, H., Marquis, C., Halliday, G., & Kwok, J. B. (2023). Single-cellDNA methylation sequencing by combinatorial indexing and enzymatic DNAmethylation conversion. Cell & Bioscience, 13(1), 2.https://doi.org/10.1186/s13578-022-00938-9
Daskeviciute, D., Chappell-Maor, L., Sainty, B., Arnaud, P.,Iglesias-Platas, I., Simon, C., Okae, H., Arima, T., Vassena, R.,Lartey, J., & Monk, D. (2025). Non-canonical imprinting, manifesting aspost-fertilization placenta-specific parent-of-origin dependentmethylation, is not conserved in humans. Human Molecular Genetics,34(7), 626–638. https://doi.org/10.1093/hmg/ddaf009
Edwards, C. A., Watkisnon, W. M., Telerman, S. B., Hulsmann, L. C.,Hamilton, R. S., & Ferguson-Smith, A. C. (2023). Reassessment of weakparent-of-origin expression bias shows it rarely exists outside of knownimprinted regions. In eLife. https://elifesciences.org/articles/83364;eLife Sciences Publications Limited.https://doi.org/10.7554/eLife.83364
Goyal, R., Reinhardt, R., & Jeltsch, A. (2006). Accuracy of DNAmethylation pattern preservation by the Dnmt1 methyltransferase.Nucleic Acids Research, 34(4), 1182–1188.https://doi.org/10.1093/nar/gkl002
Greenberg, M. V. C., Glaser, J., Borsos, M., Marjou, F. E., Walter, M.,Teissandier, A., & Bourc’his, D. (2017). Transient transcription in theearly embryo sets an epigenetic state that programs postnatal growth.Nature Genetics, 49(1), 110–118. https://doi.org/10.1038/ng.3718
Haig, D., & Mainieri, A. (2020). The Evolution of<span class="nocase">Imprinted microRNAs</span> and Their RNA Targets.Genes, 11(9), 1038. https://doi.org/10.3390/genes11091038
He, C., ZHAO, B. S., NARKHEDE, P., Liu, C., & CUI, X. (2021). Methodfor highly sensitive DNA methylation analysis (Patent US11130991B2).
Hernandez Mora, J. R., Buhigas, C., Clark, S., Del Gallego Bonilla, R.,Daskeviciute, D., Monteagudo-Sánchez, A., Poo-Llanillo, M. E., Medrano,J. V., Simón, C., Meseguer, M., Kelsey, G., & Monk, D. (2023).Single-cell multi-omic analysis profiles defective genome activation andepigenetic reprogramming associated with human pre-implantation embryoarrest. Cell Reports, 42(2), 112100.https://doi.org/10.1016/j.celrep.2023.112100
Jadhav, B., Monajemi, R., Gagalova, K. K., Ho, D., Draisma, H. H. M.,van de Wiel, M. A., Franke, L., Heijmans, B. T., van Meurs, J., Jansen,R., Hoen, P. A. C. ‘t, Sharp, A. J., Kiełbasa, S. M., GoNL Consortium, &BIOS Consortium. (2019). RNA-Seq in 296 phased trios provides ahigh-resolution map of genomic imprinting. BMC Biology, 17(1), 50.https://doi.org/10.1186/s12915-019-0674-0
James. (2014). 5mC-PCR: Preserving methylation status during polymerasechain reaction. In Enseqlopedia.
Kindlova, M., Byrne, H., Kubler, J. M., Steane, S. E., Whyte, J. M.,Borg, D. J., Clifton, V. L., & Ewing, A. D. (2023). An allele-resolvednanopore-guided tour of the human placental methylome (p.2023.02.13.528289). bioRxiv. https://doi.org/10.1101/2023.02.13.528289
Laird-Offringa, I. A., Asong, J., Campan, M., Chen, P.-H., Marconett, C.N., & Haworth, I. S. (2016). Accurate in vitro copying of dnamethylation (Patent US20160130643A1).
Li, L., Guo, F., Gao, Y., Ren, Y., Yuan, P., Yan, L., Li, R., Lian, Y.,Li, J., Hu, B., Gao, J., Wen, L., Tang, F., & Qiao, J. (2018).Single-cell multi-omics sequencing of human early embryos. Nature CellBiology, 20(7), 847–858. https://doi.org/10.1038/s41556-018-0123-2
Liu, C., Cui, X., Zhao, B. S., Narkhede, P., Gao, Y., Liu, J., Dou, X.,Dai, Q., Zhang, L.-S., & He, C. (2020). DNA 5-Methylcytosine-SpecificAmplification and Sequencing. Journal of the American ChemicalSociety, 142(10), 4539–4543. https://doi.org/10.1021/jacs.9b12707
Monk, D., Morales, den Dunnen, Russo, Court, Prawitt, Eggermann, Beygo,Buiting, Tümer, & and. (2018). Recommendations for a nomenclature systemfor reporting methylation aberrations in imprinted domains.Epigenetics, 13(2), 117–121.https://doi.org/10.1080/15592294.2016.1264561
Okae, H., Chiba, H., Hiura, H., Hamada, H., Sato, A., Utsunomiya, T.,Kikuchi, H., Yoshida, H., Tanaka, A., Suyama, M., & Arima, T. (2014).Genome-Wide Analysis of DNA Methylation Dynamics during Early HumanDevelopment. PLoS Genetics, 10(12), e1004868.https://doi.org/10.1371/journal.pgen.1004868
Rajput, S. K., Kumar, S., Dave, V. P., Rajput, A., Pandey, H. P., &Datta, T. K. (2012). An Improved Method of Bisulfite Treatment andPurification to Study Precise DNA Methylation from as Little as 10 pgDNA. Applied Biochemistry and Biotechnology, 168(4), 797–804.https://doi.org/10.1007/s12010-012-9820-7
Tanaka, K., & Okamoto, A. (2007). Degradation of DNA by bisulfitetreatment. Bioorganic & Medicinal Chemistry Letters, 17(7),1912–1915. https://doi.org/10.1016/j.bmcl.2007.01.040
Tucci, V., Isles, A. R., Kelsey, G., Ferguson-Smith, A. C., Tucci, V.,Bartolomei, M. S., Benvenisty, N., Bourc’his, D., Charalambous, M.,Dulac, C., Feil, R., Glaser, J., Huelsmann, L., John, R. M., McNamara,G. I., Moorwood, K., Muscatelli, F., Sasaki, H., Strassmann, B. I., …Ferguson-Smith, A. C. (2019). Genomic Imprinting and PhysiologicalProcesses in Mammals. Cell, 176(5), 952–965.https://doi.org/10.1016/j.cell.2019.01.043
Yi, S., Long, F., Cheng, J., & Huang, D. (2017). An optimized rapidbisulfite conversion method with high recovery of cell-free DNA. BMCMolecular Biology, 18(1), 24.https://doi.org/10.1186/s12867-017-0101-4
Zhu, P., Guo, H., Ren, Y., Hou, Y., Dong, J., Li, R., Lian, Y., Fan, X.,Hu, B., Gao, Y., Wang, X., Wei, Y., Liu, P., Yan, J., Ren, X., Yuan, P.,Yuan, Y., Yan, Z., Wen, L., … Tang, F. (2018). Single-cell DNA methylomesequencing of human preimplantation embryos. Nature Genetics, 50(1),12–19. https://doi.org/10.1038/s41588-017-0007-6
Zink, F., Magnusdottir, D. N., Magnusson, O. T., Walker, N. J., Morris,T. J., Sigurdsson, A., Halldorsson, G. H., Gudjonsson, S. A., Melsted,P., Ingimundardottir, H., Kristmundsdottir, S., Alexandersson, K. F.,Helgadottir, A., Gudmundsson, J., Rafnar, T., Jonsdottir, I., Holm, H.,Eyjolfsson, G. I., Sigurdardottir, O., … Stefansson, K. (2018). Insightsinto imprinting from parent-of-origin phased methylomes andtranscriptomes. Nature Genetics, 50(11), 1542–1552.https://doi.org/10.1038/s41588-018-0232-7
In which case we need a donor for the placenta. ↩︎
“At 2 weeks of postnatal age, male and female paternal-knockout micewere visibly smaller, with a 20% weight reduction (Fig. 6a,b,d,e) thataffected all organs uniformly (Supplementary Fig. 8c). The unimodaldistribution of the body weight data into 10% bin groups was consistentwith high penetrance and low variance of the phenotype (Fig. 6f), butwith a stronger effect in females (Fig. 6a–d). The undergrowth phenotypetended to minimize with age, but it was nevertheless persistent, asmeasured at 30 weeks (Fig. 6c).” (Greenberg et al., 2017) ↩︎
Unless we have really good reasons to believe that a particularimprint is not crucial for development. ↩︎
So far I know of only 2 paternal imprints where I am confident thatthey are not only established after fertilization and those are theIGF2/H19 DMR and the IG-DMR. There are probably more paternal imprints,that are important, but erased early on in development (possibly true ofRASGRF1 in humans). ↩︎
Phi29 is already a common choice in Preimplantation embryonictesting and is used in commercial Kits like Repli-G fromQuiagen. ↩︎
He et al. (2021) ↩︎
Although one can’t strictly separate replications with Phi29, sinceit is working continuously, but in the paper they claim to haveamplified their 10pg sample by 100x. ↩︎
Because DNMT1 alone has a tendency to not only methylatesemi-methylated, but also unmethylated cytosine (Goyal et al., 2006;James, 2014). We might also want to add UHRF1, which Laird-Offringa etal. (2016) claims significantly helps with this problem. They tried thisin E. coli which do not have histones. If it works in E. coli my guessis that it should also be helpful during PCR. ↩︎
See table 2 in the paper’s appendix. Also for some reason their100ng control ends up with lower genomic coverage than their 4ngcontrol? Even though the 100ng control has a higher mapping ratio? ↩︎
I think this is why rodents turn off their X-chromosomestwice, buthonestly I still don’t quite understand why this happens. ↩︎
You can find them on AWS by running
aws s3 ls s3://1000g-ont/ --recursive | grep ".bed" | grep -P "mat|pat"in the shell with AWS-cli installed. ↩︎
Discuss