Alzheimer’s disease is characterized by the abnormal formation of amyloid-beta peptide plaques and tau tangles in the brain. Although initially identified in 1907, the molecular structures and arrangements of these protein aggregates remain unclear. Now, a research team headed up at the University of Leeds has determined the 3D architecture of these molecules within a human brain for the first time, reporting the findings in Nature.
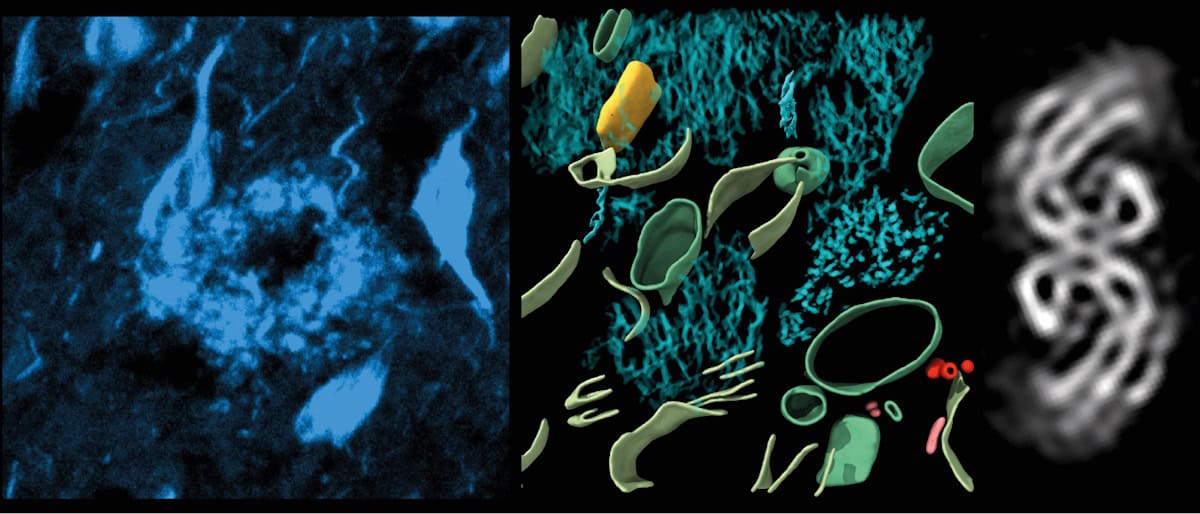
The researchers used cryo-electron tomography (cryo-ET) techniques to create 3D maps of tissues in a postmortem Alzheimer’s disease donor brain. They revealed the molecular structure of tau in brain tissue and the arrangement of amyloids, and identified new structures entangled within these pathologies.
“[These] detailed 3D images of brain tissue…for the first time bring clarity to the in situ organization of amyloid-beta and tau filament,” states Sjors Scheres, of the MRC Laboratory of Molecular Biology, in an accompanying commentary article.
Scheres explains that the research team had to overcome several major hurdles, including slicing thin enough brain tissue samples for electrons to pass through, freezing hydrated samples fast enough to prevent crystallization that can interfere with the cryo-ET imaging, and identifying relevant areas containing amyloid-beta and tau tangles to image.
For the study, lead author René Frank and colleagues examined freeze-thawed postmortem brain samples of the mid-temporal gyrus from an Alzheimer’s disease donor and a healthy donor. To identify areas of amyloid-beta and tau, they thawed the samples, sliced the brain tissues into 100–200 μm slices and added methoxy-X04 (a fluorescent dye that binds amyloid), before rapidly refreezing the samples.
The researchers then performed cryo-ET on 70-nm-thick tissue cryo-sections from a dye-labelled amyloid-beta plaque and a location enriched in tau tangles and threads. Using cryo-fluorescence microscopy to guide the cryo-ET, they acquired images from different angles and used these to computationally reconstruct a tomographic volume. They collected 42 tomograms in and around regions of amyloid-beta, 25 tomograms in regions containing tau tangles, plus 64 tomograms from the healthy brain tissue as controls.
To obtain higher-resolution structural information, the researchers picked subvolumes containing filaments for alignment and averaging. Subtomogram averaging of 136 tau filaments from a single tomographic volume generated the in situ structure of tau with 8.7 Å resolution.
The researchers report that the amyloid-beta plaques had a lattice-like architecture of amyloid fibrils interspersed with non-amyloid constituents, including extracellular vesicles, fragments of lipid membranes and unidentifiable cuboidal particles. Because these non-amyloid constituents were not present in healthy brain samples, they suggest that they are also a component of Alzheimer’s pathology, and may be related to amyloid-beta biogenesis or a cellular response to amyloid.
The amyloid-beta plaques also contained branched amyloid fibrils and protofilament-like rods. The team speculates that these branched fibrils and rods may contribute to the high local concentration of amyloid-beta that characterizes plaques.
Frank and colleagues also identified tau clusters within cells and in extracellular locations. The tau filaments were unbranched and arranged in parallel clusters. They observed both paired helical filaments and straight filaments, which did not mix randomly with each other, but tended to be close to filaments of the same type, often arranged with the same polarity. The researchers suggest that the non-random arrangement may be caused by interactions between filaments or growth in parallel from neighbouring focal points.
The collaboration – also including researchers at Amsterdam UMC, the University of Cambridge and Zeiss Microscopy – represents new efforts by structural biologists to study proteins directly within cells and tissues, to determine how proteins work together and affect one another, particularly in human cells and tissues affected by disease.
“The approaches for obtaining 3D molecular architectures and structures of human tissues with cryo-CLEM [cryo-correlated light and EM]-guided cryo-ET in Alzheimer’s disease sets the ground for interrogating other common dementias and movement disorders,” says Frank. “These include frontotemporal dementia, amyotrophic lateral sclerosis (motor neuron disease) and Parkinson’s disease.”
The post Cryo-electron tomography reveals structure of Alzheimer’s plaques and tangles in the brain appeared first on Physics World.

